Last Friday, November 7, I spoke at Virginia Mason Medical Center’s Grand Rounds on the topic of “Lung Cancer in Non-Smokers.” Grand Rounds is a common teaching tool in medical facilities that helps healthcare providers stay current and provide the best possible care. In our one-hour session, my pulmonologist Dr. Steven Kirtland talked about the epidemiology of non-smoker lung cancer (its frequency, possible causes, patient demographics), I shared my epatient story, and my oncologist Dr. Joseph Rosales talked about lung cancer mutation testing and targeted therapies. You can see my 20-minute presentation below.
———————————————–

© Janet Freeman-Daily
In Seattle, home of Starbucks, everyone drinks coffee. Can YOU tell which of them has lung cancer? In this picture, it’s the person on the far right: me.
In March 2011, I was healthy, a bit overweight, and exercising regularly. However, I’d had a nagging cough for a few months. To make my husband happy, I mentioned the cough to our doctor. Two months, two rounds of antibiotics, one x-ray, and a bronchoscopy later, I spent a very anxious four days waiting for biopsy results.
When I heard, “lung cancer,” I could barely believe the diagnosis. I called my sister to tell her the news, poured a big glass of wine, and lost myself in a favorite science fiction movie.
I had never lived with smokers, never worked in a smoking environment, never smoked anything (except a salmon). I knew nothing about lung cancer. The facts I found online were not encouraging. As we moved through the various staging procedures, my family and I experienced increasing levels of fear:
- “It’s OK, it’s just one tumor. VATS surgery will probably take care of it.”
- “Well, OK, lymph nodes are involved, but still inside one lung. We can remove the lung, right?” (OMG)
- “There’s a lymph node between the lungs, severe inflammation and obstructive pneumonia. Stage 3a. No surgery.” This is serious. After my mediastinoscopy, my sister left the hospital convinced I was dying.
I was reassured to hear Dr, Rosales say he considered me curable. I was eager to start aggressive lung cancer treatment. But the universe, it seemed, objected to the treatment plan. The interior of my tumor had died and become colonized by bacteria. Even though we finally found an antibiotic that knocked out the infection, my recovery took weeks. During that time, I developed a clot on my PICC line and required daily self-injected blood thinner. Heaven forbid I should be a boring, vanilla cancer patient! I worried my lung cancer was growing while I waited to start treatment.
I hit bottom a few days after my second bronchoscopy. I awoke at 3 AM coughing up a lot of blood, and Dr. Kirtland told me to go to the ER. I was released later that morning, just in time to drive 30 miles to my first radiation treatment. The linear accelerator was down two hours for repairs, but I did eventually get zapped. My husband and I drove to a nearby restaurant for a very late lunch, and came out to find our car had a flat tire. Not a very reassuring start.
The next few months revolved around my daily appointments. Perhaps the toughest part was telling my autistic adopted son that he might lose another mother to cancer. My bucket list became laser-focused on helping him prepare to live on his own. Despite fatigue and severe esophagitis, I was able to attend my niece’s wedding a month later. You haven’t lived until you’ve had Ahi tuna encrusted in coffee beans–pureed for a liquid diet. At one point I was taking ten different meds to control pain and side effects. My butt was dragging, my blood values tanked after one full dose of chemo, and I broke out in hives during my second red cell transfusion. But gradually, I started feeling better.
It all seemed worthwhile when my first post-treatment CT scan showed my lymph nodes had resolved and the primary tumor had shrunk about 90%. I wanted that tumor OUT, if possible. I had 15 appointments in 16 days to determine if the surgery would be an acceptable risk–we only had a short window in which to do surgery before radiation changes would make it too risky. Juggling that schedule generated a lot of additional stress — my family’s life revolved completely around my cancer. I wished Virginia Mason had a Lung Cancer Navigator to coordinate all the appointments between seven different professionals at four different facilities, communicate results, explain terms and options in more detail, and ensure timely follow-up. The last procedure, a PET scan, showed a hot spot on my collarbone. Dr. Kirtland quickly arranged an MRI scan for the next morning, and a surgical open biopsy on the following day. To find the tiny suspicious lymph node, the surgeon used an innovative combination of FDG tracer and a Geiger counter. Two nodes contained cancer.

I was now a metastatic lung cancer patient. The panic bowled me over like a 50-foot wave. Alone at home, I became a puddle of hopelessness–for about an hour. Then I shifted gears and got busy asking questions in an online lung cancer forum. The support I received there was essential for maintaining hope while I processed my new diagnosis. They helped me accept there was no point undergoing a risky lung surgery with a tough recovery when it wouldn’t cure me.
Together Dr. Rosales and I decided to start a new chemo after a couple of months, to give me time to recover from my first line treatment. I appreciated that he listened to my concerns about the delay, and that he was careful not to give me an expiration date that might take away hope. I didn’t want to die before applying for my Boeing pension, so I asked how long I had left. Dr. Rosales estimated about two years.
In the next ten weeks, my mother died, I started taking prednisone for radiation pneumonitis, and a new three-inch tumor grew very visibly on my collarbone. My extended family gathered for what we thought might be my last Thanksgiving. I had no desire to celebrate Christmas that year. My most memorable gifts were a newly-installed power port and a hint that my hair was coming in curly.
In my online lung cancer forum, I learned about a clinical trial called the Lung Cancer Mutation Consortium Protocol. It tested lung cancer tumor tissue for mutations in ten different genes. I consulted with my Virginia Mason doctors, but they hadn’t heard of it. I found the trial listing on clinicaltrials.gov, then contacted the trial sites until I found one accepting patients. The University of Colorado Cancer Center agreed to test my existing biopsy samples even though I could not fly to Denver due to concerns my hollow primary tumor might cause a pneumothorax. My entire team was disappointed when all tests were negative. I continued networking with experienced lung cancer patients, and when Dr. Rosales and I discussed chemo options, I suggested Avastin based on some new research. We mutually agreed on Alimta plus Avastin–he was willing to be more aggressive in my treatment because he knew I understood the risks.
Ten days after I started the new chemo in January, my collarbone tumor was visibly shrinking. I was extremely encouraged despite a sudden worsening of my pneumonitis and my new appreciation for ‘roid rage. Still, I was glad to finish chemo after six rounds–I was losing my voice frequently, and towards the end I felt like I always had the flu. I began to understand how some people could decide to stop cancer treatment. But I couldn’t argue with the results: all the original tumors were gone, the new tumor had shrunk 90%, and no new tumors appeared. We decided to treat this one remaining tumor as an oligo-recurrence and go for a possible cure — radiation therapy might knock my cancer out for good. My skin burned raw, but I made it through.
The next PET scan showed no activity around my collarbone. Yay! However…it also showed two new nodules in my “good” lung, both outside the radiation field. Seems I progress whenever I stop chemo. Another bronchoscopy was scheduled two weeks out, after my husband and I returned from a weekend with my nephew in Denver.

© University of Colorado Cancer Center (used with permission)
Here’s where the tone of my story changes.
Months before, one of my online lung cancer friends told me of a new mutation called ROS1. I fit the profile of typical patients who had it, and a Phase 1 ROS1 trial still had slots left, but only a lab in Boston could test for it. No one at Virginia Mason knew about it. On my last full day in Denver, I realized the University of Colorado Cancer Center was not far from my nephew’s house. I might be able to personally thank the people who had helped me get my previous mutation testing done. I sent an email Sunday afternoon, and was amazed to get an email back that evening saying I could meet the next day with Dr. Bunn, the Center’s Director. He told me they could now test for additional mutations, including ROS1. I gave him permission to test my remaining slides.
A week later, Dr. Kirtland performed a bronchoscopy on the larger of my new nodules. He got a good sample, but couldn’t find any cancer cells. The biopsied nodule could be inflammation, BOOP, or cancer. The other nodule was too small to biopsy.
The very next day, Dr. Bunn emailed me to say I had “an impressive ROS1 rearrangement” and University of Colorado had an opening in a crizotinib trial for me, if I wanted it. Crizotinib is a twice-daily pill that targets cells expressing certain mutations, including ROS1. It produced a terrific response rate in the initial trial with substantially fewer side effects than chemo for most patients. He also said I could join the trial later if I didn’t have active cancer now. I was so excited that I almost screwed up forwarding the email to Dr. Rosales.
The following morning, Dr. Rosales called, also excited by my ROS1 news. If the new nodule was cancer, he agreed I should enter the ROS1 trial rather than start taking Alimta.
That afternoon, Dr. Kirtland called. He had taken my case to the Tumor Board, and their consensus said the biopsied nodule was radiation changes. I was to restart prednisone. (My husband asked, “What will he give ME when YOU restart steroids?”) In a month I would have a CT to determine if the nodules responded to prednisone, or continued growing. I’d come to accept that living with stage IV lung cancer brought uncertainties, but that didn’t make the waiting easier.
The CT scan showed the larger nodule had not changed, but the smaller nodule had grown nearly fifty percent. The good news was that I could once again ramp down off prednisone. The bad news was that the smaller nodule was likely cancer–I needed to either restart Alimta, or join the crizotinib trial.

I was on the phone the next morning to the University of Colorado, inquiring about how to join the ROS1 trial. Their doctors said I might be able to join the trial without having another biopsy. Virginia Mason medical records and radiology really hustled to pull my records together. After four days, I was flying to Denver with the intention of staying until I was accepted into the trial, and wondering why the heck I was traveling a thousand miles away from my home and family to try an experimental cancer treatment that might not work. My concerns were not eased by the delays caused by Hurricane Sandy, which shut down the trial sponsor Pfizer’s headquarters in New York City during my screening period. My acceptance into the trial came at the last possible minute.
I took my first crizotinib pill two years ago last Thursday. My first scan eight weeks later showed both nodules were gone, indicating they likely were both cancer. As of last Monday’s scan, I have had No Evidence of Disease (known to cancer patients as “NED”) for 22 months and counting. I may be able to stay on this drug for months or years longer. Yet targeted therapies like the one I take do not offer a permanent cure. In time I’ll probably develop resistance to the drug. There IS no cure for metastatic lung cancer. No one can say how long I will live. Sometimes that weirds me out. Yet I’m hopeful that when this trial drug stops working, another clinical trial will be a good match for me.

It’s an odd existence, living from scan to scan in eight-week increments. I still sometimes experience scanxiety, as we patients call it. I often hide out in the bedroom for days before a scan so my scanxiety doesn’t bite anyone. There is no logical reason for this feeling. My scans have been clean for months, and I have no symptoms that would indicate the next scan should be any different. If I do have a recurrence, I know I have some treatment options. Even if I had no treatment options, I am not afraid of dying. Apparently my subconscious simply overpowers my conscious positive thoughts. It probably doesn’t help that whenever I’m leaving for a scan, my son hugs me hard and says, “Please don’t die Please don’t die Please don’t die.”
Several events conspired to give me severe scanxiety a year ago. It felt like a panic attack. Not only was the timing near the anniversaries of my two cancer recurrences, but a friend on a targeted therapy had developed brain mets weeks after a brain MRI, a neighbor had died when her lung cancer spread to her brain covering, and the online ROS1 buddy who had first told me about my clinical trial appeared to be progressing after two years on crizotinib. A network of lung cancer patients provides invaluable support, but it requires accepting that friends will die frequently.

I feel overwhelmingly grateful for everything and everyone that has helped me survive as long as I have: medical science that discovered new ways to treat my condition, compassionate healthcare providers at Virginia Mason and in Denver, insurance that paid for most of my care, family and friends who supported me, a knowledgeable online lung cancer community, and all the prayers and good wishes lifting me up throughout my cancer journey. I’m acutely aware that many lung cancer patients do not have these supports and opportunities.
Being given a second chance at life, however long it might be, tends to give one a different perspective. Seeing the sunset paint Mount Rainier fills my heart. Chatting with my sister over a latte keeps me smiling for a week.
A second chance at life also makes one introspective. Why was I spared when others died? Why does my mutation have an effective treatment when others don’t? Why am I able to see one of the best lung cancer doctors in the world when many patients can’t afford proper treatment? Why am I still here?
I had been blessed with gifts that helped me survive my cancer journey thus far. In my previous career of aerospace engineering, I was a “translator” of sorts: I researched science and technology developments and helped others understand their benefits. Thanks to these skills, I’m able to understand lung cancer treatments and research. I’m able to explain what I’ve learned, both verbally and in writing, in everyday terms. And I’m able to advocate for myself with healthcare providers.
I have chosen to use these gifts to help other lung cancer patients by going public with my lung cancer in my blog, in online forums, and in public speaking. Most patients don’t know about the new treatments like the one I’m taking–even some doctors don’t know. Lung cancer patients need more than compassion. They need information about second opinions, mutation testing, side effects, treatment options, and clinical trials. They need HOPE.

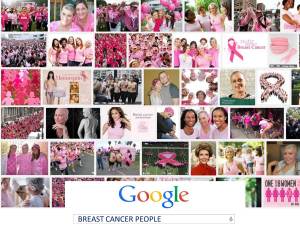
Going public has also helped more people understand that ANYONE with lungs can get lung cancer—and NO ONE deserves to die from it. Lung cancer kills about as many people as the other top four cancers combined, yet it receives fewer federal research dollars per death than any of them. Why is that? Are lung cancer patients not worth saving? The answer becomes clear when you google the words “lung cancer people.” No throngs of ribboned supporters; few smiling survivors. You see diseased lungs, death … and smoking. Lung cancer has an image problem. The first question I hear when I mention my disease is: “Did you smoke?” People blame patients for getting lung cancer. The breast cancer community has changed how the world sees their disease. The lung cancer community must do the same. We’ve all done things that impact our health. Yes, it’s healthier not to smoke. But it’s not a sin that warrants the death penalty.
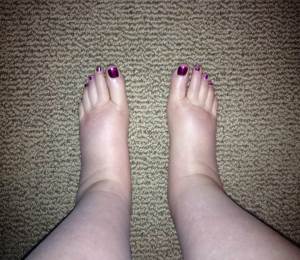
© Janet Freeman-Daily
Precision medicine allows me to live with lung cancer as a chronic illness instead of a death sentence. True, it’s not the same life I had Before Cancer. I can’t do the active sports that I used to do. Chemotherapy left me with peripheral neuropathy and cognitive changes. Radiation scarred my lungs and damaged the nerve bundle for my right arm. A year of steroids packed on the fat while decreasing muscle tone. Crizotinib causes edema and graces me with antisocial gut behaviors. Some combination of side effects keeps my red blood cell count just below normal. When I exercise on the treadmill, I can’t get manage a brisk walk for more than 30 seconds without breathing fast and hard.

Image Credit: Stephanie Jarstad
I’m not complaining, mind you–I’m happy to be alive and have a relatively normal life on targeted therapy. It even allowed me to play a casual game of softball in Cheney Stadium at my 40th high school reunion. The moment I put the glove on my left hand, my body recalled those years on the softball diamond. After some initial fumbles, I could catch, throw, pitch and hit. And I got to first base before the ball did. I could not have even reached first base while on chemo.
As a three-year lung cancer survivor, I’ve already lived beyond my prognosis. I will stay with targeted therapy and other clinical trials as long as my quality of life makes it worthwhile. Lung cancer research has found more new treatments in the past few years than ever before, and the pace of discoveries is accelerating. As people begin to realize that ANYONE can get lung cancer (including never smokers like me), the stigma will hopefully begin to fade, and research funding will increase.
We lung cancer patients deserve hope, and a cure. Every one of us.